Crosstalk on the atrial channel
Tracing
Manufacturer Medtronic
Device PM
N° 17
Patient
74-year-old man implanted with an Ensura DR dual-chamber pacemaker for complete atrioventricular block after aortic valve surgery; atrial lead positioned on the right atrium, ventricular lead positioned on the upper septum; asymptomatic patient, control visit 3 months after implantation.
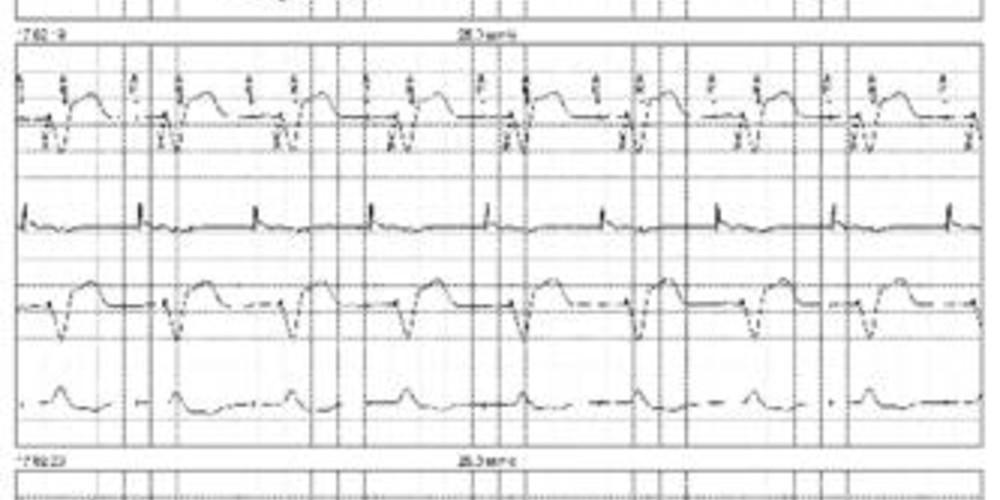
Graph and trace
Tracing 17a: tracing recorded at patient arrival; programming in DDDR mode; the first line corresponds to lead I on which the markers are superimposed, the second line corresponds to the atrial EGM, the third line to lead II and the last line to lead III;
- AP-VP-AR sequences with fixed VP-AR intervals; atrial pacing appears to be effective; the atrial EGM shows the atrial depolarization events according to the different APs confirming an effective atrial capture; effective ventricular capture; following each VP, the atrial EGM shows a small signal probably corresponding to ventricular crosstalk on the atrial channel;
Tracing 17b: change in programming with prolongation of the post-ventricular atrial blanking;
- same tracing and programming as above;
- change in programming with prolongation of the blanking (programming of partial blanking);
- the atrial signal sensed after each ventricular pacing is identical but is now sensed during the post-ventricular atrial blanking period (fixed VP-Ab intervals);
Tracing 17c: modification in programming (partial blanking to partial plus);
- same tracing and programming as above;
- change in programming (partial blanking plus);
- disappearance of crosstalk.
Other articles that may be of interest to you







Post-ventricular atrial blanking is the first component of the post-ventricular atrial refractory period. Traditionally, no sensing was possible in a window identified as a blanking which constituted an absolute refractory period. On the new pacemaker platforms, this differentiation between blanking (absence of sensing and absence of visualization of the signal on the chain of markers) and refractory periods (sensing possible with visualization on the chain of markers) is less clear-cut. Indeed, according to the programming, an atrial event sensed during post-ventricular atrial "blanking" can be visualized on the chain of markers and interfere with the functioning of different algorithms. There are 3 programming options:
A signal detected in the PVAB (classified as Ab) likewise to a signal detected in the PVARP (classified as AR) does not trigger an AV delay but is counted for the diagnosis of atrial arrhythmias.
In this patient, the diagnosis of crosstalk is evident. The sensed supernumerary atrial signal is small in size and very precocious relative to the ventricular pacing. To avoid this crosstalk, 4 solutions can be envisioned: