Induction of VF with flawed detection causing shock diversion
This 40-year-old man underwent implantation of a Boston Science Incepta single chamber defibrillator in the context of spontaneous, type 1 Brugada syndrome and a history of syncope. The ventricular lead was implanted in the septum with an average sensing amplitude of 3.5 mV during sinus rhythm. VF was induced with the ventricular sensitivity specifically programmed for this type of procedure at 1.0 mV.
Episode summary
An episode of VF was induced. Despite the detection of an event in the VF zone, the first therapy, consisting of a 21-J shock, was diverted because of failure of reconfirmation and the attempt was abandoned. The arrhythmia was redetected and a 21-J shock was delivered after a very short charge time, since the capacitors were previously charged and the therapy was abandoned.
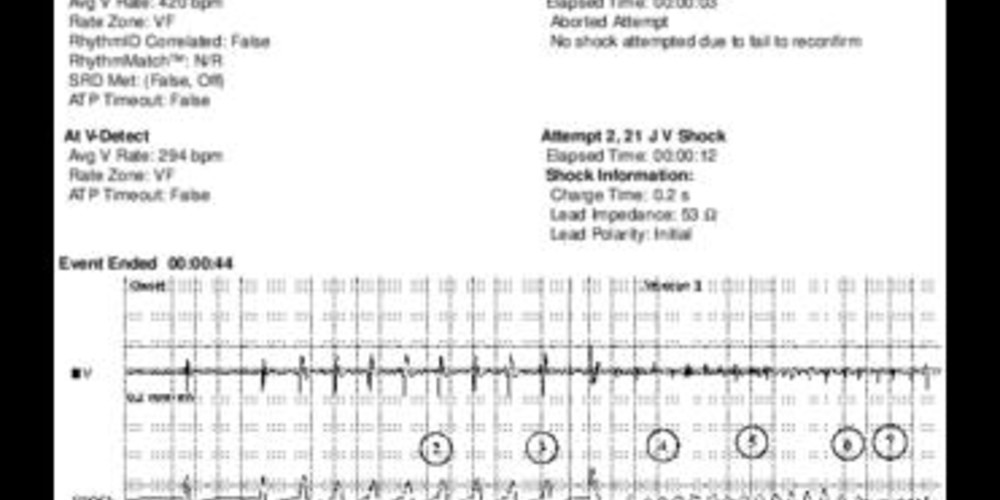
Tracing
- very short charge to deliver a shock on the T wave; 2 very close markers are visible at the beginning and end of the charge;
- 8 stimuli delivered at a rate of 200 bpm;
- low-amplitude shock delivered on the T wave; a 500-ms refractory period followed the shock, during detection was precluded in both chambers;
- after the refractory period, the first ventricular event was not counted (--);
- trigger of a very low voltage polymorphic tachyarrhythmia;
- after 8 consecutive cycles in the VF zone and fulfillment of the 8 out of 10 criterion of the detection window, an episode was detected (V-Epsd);
- first undersensed ventricular EGM;
- after the 1-sec duration, a sustained episode (V-Detect) was detected; onset of the charge of the capacitors;
- end of the charge;
- in absence of reconfirmation after the end of the charge, the shock was diverted (Dvrt) by the device because the 2 out of 3 short cycles criterion was not fulfilled; ventricular undersensing was responsible for the erroneous diagnosis of spontaneous termination of the arrhythmia;
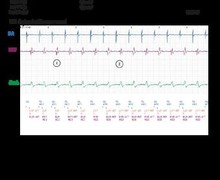
- further detection of an 8 out of 10 short cycles window (no marker is visible on the tracing); onset of the post-redetection duration;
- detection of another episode in the VF zone (8 out of 10 criterion in the VF zone and post redetection duration fulfilled). Three durations were programmable: 1) the initial duration, independently programmable in the VF and in the VT zones, 2) the post-redetection duration (when a charge has been diverted or after a burst of ATP; it is programmable in the VT zone, though is fixed at 1.0 ms in the VF zone), and 3) the post-shock duration (it is programmable in the VT zone, though is fixed at 1.0 ms in the VF zone);
- another very short charge, since the previous charge had not had the time to dissipate;
- this electrical shock delivered at the end of the 500-ms period; when a charge has been diverted, for a same episode, the following charge must be delivered, because of concern for ventricular undersensing leading to the device mistakenly calling the VF episode terminated;
- successful shock and termination of the arrhythmia.
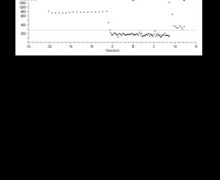
Graph
This graph displays the characteristics of intermittent undersensing of VF with a succession of short cycles in the VF zone (16) and of longer cycles hovering around the sinus zone (17), evidence of these detection gaps.





As explained before, the reliable detection of arrhythmias is an indispensable prerequisite for the treatment of VF by electrical shocks. During induction, the presentation is straightforward in 2 instances: 1) sensing is flawless throughout the initial detection, the duration and the charge of the capacitors, and the programmed safety margin of the sensitivity is considered satisfactory, or 2) sensing is poor, with so many gaps, that the initial detection criteria are never fulfilled or the charge is systematically diverted and no therapy can be delivered; in these circumstances the lead position must be changed to obtain a satisfactory level of sensitivity. Occasional patients, as in this case, are more challenging to manage. Indeed, during this induction, the operator observed very few failures of ventricular sensing but at the worst time, i.e. the confirmation at the end of charge. Indeed, the number of undersensed R waves is important but more importantly the timing of this undersensing is crucial. The first charge was diverted and the shock delivery was delayed by a few seconds. This example justifies the fact that a charge cannot be diverted twice during the same episode. The graph shows more clearly sensing dropout, facilitated by the variability of the signals amplitude, which mislead the automatic sensitivity algorithm. In this patient, the signals during VF were fragmented and low in amplitude, also facilitating undersensing. During sinus rhythm, ventricular sensing, measured at 3.5 mV, was of average size. Despite the successful electrical shock, the lead was repositioned from the right ventricular apex toward the high septum, which slightly increased the ventricular signal amplitude to 4.4 mV during sinus rhythm. Furthermore, a repeat induction procedure confirmed the reliable sensing during VF and successful defibrillation.