Basic concepts
Biventricular resynchronization offers considerable clinical benefits, including reverse remodeling with decrease in cardiac volumes, and a decrease in morbidity and mortality in patients suffering from heart failure with wide QRS complex on the surface electrocardiogram. The main limitation of this therapy is a high percentage of non-responders to the therapy. Various approaches have been proposed to lower that percentage. Once the device has been implanted, the quality of its programming is an important determinant of the quality of the response. CRT aims at changing the sequence of activation in presence of electrical conduction disorders by adjusting the activation delays among the right atrial, RV and LV leads. Two settings can be programmed in this context: 1) the AV delay that determines the activation timing between right atrium and right ventricle, with independent programming of a sensed AV delay, after sensing of a spontaneous atrial event (AS cycle-BV) and a paced AV delay following a paced atrial event (AP-cycle BV). A variable AV delay that decreases in parallel with an increase in heart rate can be programmed; 2) The VV delay is the interval between right and left ventricular activation; simultaneous activation (VV delay = 0), right pre-activation (RV à LV, X ms) or a left pre-activation (LV à RV, X ms) are programmable; a VV delay that varies between rest and exercise cannot be programmed. Short-term hemodynamic studies have found a considerable gain achievable by the optimization of the AV delay, the VV delay or both. The long-term confirmation of this benefit with respect to cardiovascular endpoints (heart failure hospitalization, death or need for heart transplantation) is still lacking.
AV delay optimization
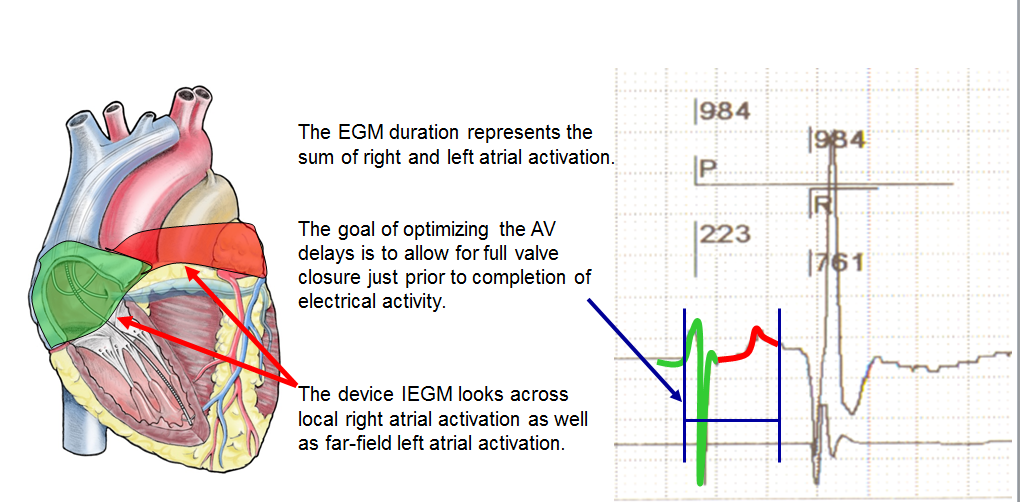
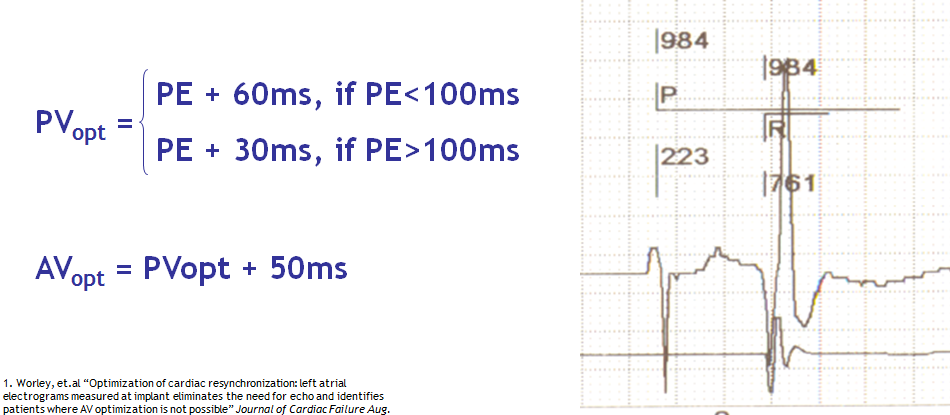
The atrial contraction contributes 20-30% of the cardiac output at rest in patients suffering from heart failure with systolic dysfunction, a contribution that increases with exercise. Patients suffering from heart failure and electrical conduction disorders often present with AV dyssynchrony, shortening of the filling time, merging of the E and A waves and diastolic mitral regurgitation. In patients, undergoing CRT, the programming of a short AV delay advances the E wave, separates it from the A wave and lengthens the filling time. The AV delay should not be excessively short, since it amputates the A wave by closing the mitral valve. Despite modest clinical evidence, it is recommended to adjust the AV delay after implantation of a CRT device.
The inter-individual variations in intra-atrial conduction and intra-ventricular disorders are wide, causing wide variations in optimal AV delay, which theoretically justify individual programming of each device. The separately programmable sensed and paced AV delays must also be optimized independently. The AV delay is usually optimized at rest, in the supine position, during fixed heart rate, conditions that differ considerably from those of everyday life. Unlike in patients with healthy hearts, in whom the optimal AV delay shortens as the heart rate increases during exercise, the response of CRT devices to stress is not predictable since, the optimal AV delay is longer in some patients during exercise than at rest, whereas in others it is shorter. While the systematic use of an automatic AV delay reduction algorithm probably ensures a reliable capture during exercise, it is not necessarily associated with an additional hemodynamic benefit. Therefore its programming should be individualized. BiV resynchronization promotes reverse remodeling and progressively decreases the end-systolic and end-diastolic volumes over time. Therefore, the AV delay must be periodically re-optimized.
An optimal AV delay promotes a maximum contribution of the left atrial contraction to LV filling, lengthens the filling time, increases the cardiac output and minimizes mitral regurgitation. If the length of the AV delay is excessive, the atria contract too soon in diastole, limiting their contribution to ventricular filling. The atrial contraction is superimposed over early diastole, echocardiographically apparent as a fusion of E and A waves, a short filling time and persistent diastolic mitral regurgitation. If the length of the AV delay is insufficient, the ventricles contract too early, causing premature closure of the mitral valve, interrupting on-going filling and limiting the atrial contribution to ventricular filling. Echocardiography shows a premature E wave, a long filling time, split E and A waves and an A wave truncated by the mitral valve closure. The decrease in end-diastolic pressure and preload decreases the peak dP/dt and the cardiac output.
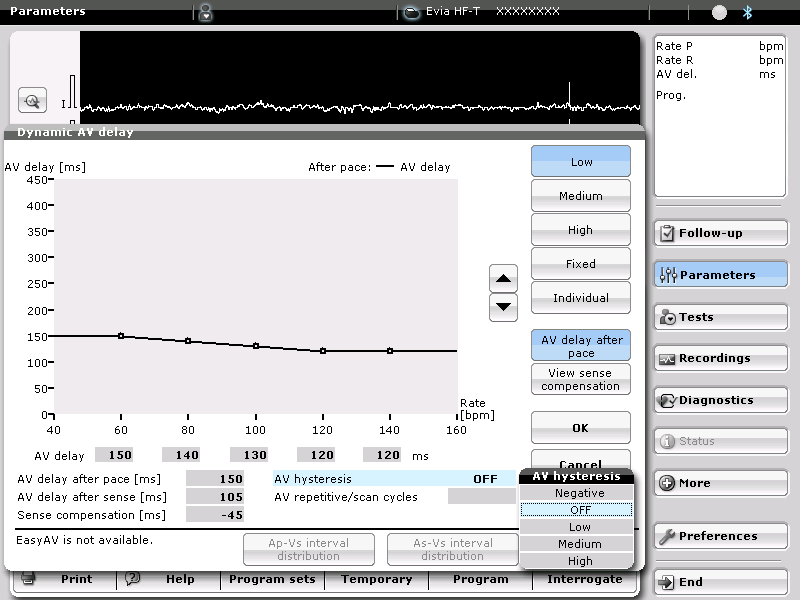
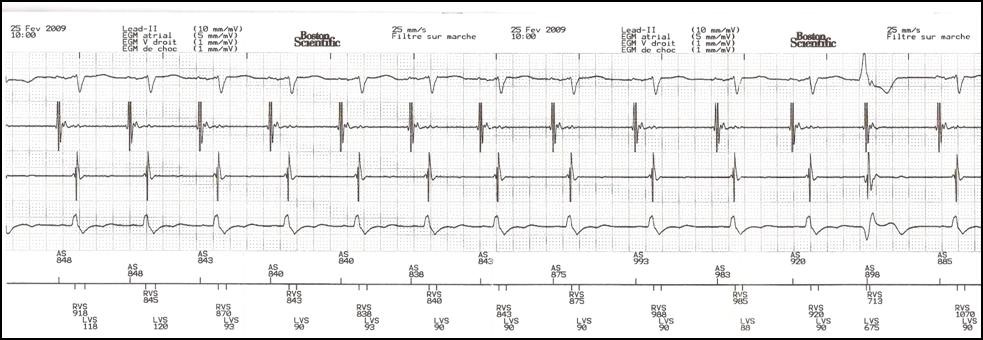
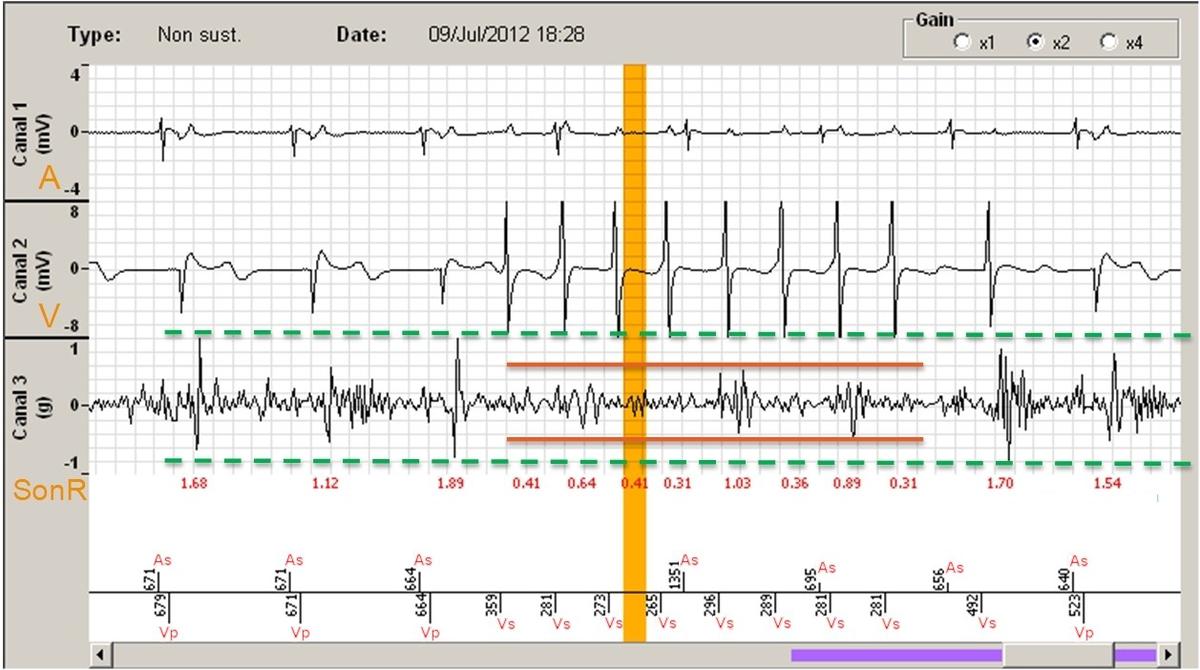
Before optimizing the AV delay, it is useful to keep in mind that, in presence of complete or high-grade AV block or a markedly prolonged PR interval, changing the AV delay has no direct effect on the amount of ventricular capture and fusion. In contrast, in presence of preserved AV conduction, prolonging the AV delay causes progressive fusion of ventricular capture with spontaneous activation. The AV delay must be adjusted under electrocardiographic guidance, remembering that, in absence of complete AV block, which is the case in the majority of patients, that adjustment varies the delay between atrial and ventricular systole, while also directly interfering with the ventricular activation sequence and the amount of ventricular fusion. This can be overcome by systematically programming a short AV delay, between 90 and 120 ms after a sensed P-wave, and between 130 and 150 ms after a paced atrial event.

Progressive adjustment of the AV delay in a CRT system recipient with preserved AV conduction. Fusion gradually increases as the AV delay lengthens.
Various means of AV delay optimization have been proposed:
- Echocardiography
A) The Ritter method, which has not been validated in a sample of patients presenting with heart failure; B) search for a maximum aortic or mitral velocity-time integral; C) search for maximum dP/dt; and D) the iterative method. The latter method, widely used in clinical practice, analyses the trans-mitral flow to obtain the longest ventricular filling time without amputation of the A wave.
- Other methods
Cardiac contractility or output can be estimated by examining the pulse wave, blood pressure, maximum dP/dt, electrocardiographic morphology, and others. The applicability of these estimates in daily practice is often limited.
- Embedded automatic optimization algorithm
If the AV delay must be optimized repeatedly and under various conditions of preload, it should ideally be accomplished by the pacemaker. Different algorithms developed by the different companies will be described at the end of this chapter.
VV delay optimization
Considerable ventricular mechanical dyssynchrony may persist after device implantation in non-responders to CRT. Adjustments of the VV delay result in sequential BiV pacing and directly modify the sequence of ventricular activation, which may alleviate the persistent dyssynchrony in non-responders. This setting may be theoretically advantageous in presence of a suboptimal position of the LV lead, or of latency and a prolonged conduction time to the site of stimulation. While the optimization of the VV delay has been associated with significant short-term hemodynamic benefits, the long-term clinical relevance of this programming remains debated and unconfirmed by clinical studies. The remodeling process probably directly influences the VV delay optimization, such that it must be re-optimized over time under various conditions of preload.
The same methods can be used to optimize the AV and VV delays. Echocardiography is often used in clinical practice. Aortic velocity-time integral, which reflects the cardiac output, maximum dP/dt, which reflects cardiac contractility, or measurement of the degree of ventricular asynchrony are also used. In light of the practical difficulties of VV optimization, its repeated automatic adjustment by the device is a promising development. However, its clinical relevance remains to be confirmed.

Influence of the VV delay on the ventricular electrical activation
Despite the evident difference in electrocardiographic morphology among the various configurations, which one is likely to be associated with an optimal clinical response is not clear.
LV alone versus BiV stimulation
The superiority of BiV over LV stimulation has not been confirmed, though short-term hemodynamic studies have consistently found a significant benefit conferred by isolated LV stimulation. Similarly, clinical studies have observed a nearly identical benefit conferred by isolated LV stimulation on NYHA functional class, exercise capacity and ventricular remodeling compared with BiV stimulation. However, all large studies demonstrating the benefits conferred by CRT have used BiV instead of LV stimulation alone. With that latter configuration, the resynchronization of the two ventricles is achieved by the fusion between the stimulated left ventricle and the spontaneous RV activation. The optimal short-term hemodynamic benefit seems to be achievable with an optimal degree of fusion, which is difficult to quantitate and preserve during exercise, due to changes in the heart rate and PR interval.
Isolated LV stimulation is an attractive option, particularly when the implanted device is a CRT-P. This can be accomplished with a dual chamber pacemaker without implanting an RV lead, which lowers the cost of the system and lowers the risk of complications. However, in pacemaker-dependent patients presenting with AV block, the implantation of a single LV lead may be risky, given the high incidence of LV lead dislodgement, rise in capture threshold, or both. In recipients of CRT-D, the implantation of a RV lead is indispensible. However, programming of the device in a “LV stimulation only” configuration eliminates the battery consumption associated with RV stimulation.